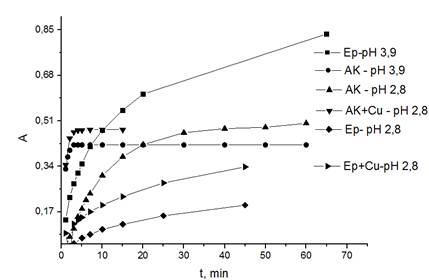
Fig. 1
Time dependence of optical density of HPC solutions with AA and EN under different conditions.СHPC = 9,76·10-5 М, СAA = СEN = 2,44·10-5 М, СCu = 9,76·10-4 М. l = 2 сm, л = 790 (рН 3,9) nm, л = 820 (рН 2,8) nm.
Zaruba S. V., Vishnikin A. B., Posudievska O. R.
Oles Honchar Dnipropetrovsk National University
STUDY OF THE OXIDATION RATE OF ASCORBIC ACID AND EPINEPHRINE BY 16-MOLYBDO-2-TUNGSTODIPHOSPHATE IN VARIOUS CONDITIONS
Epinephrine (EN), traditionally called adrenaline, is one of the important catecholamine neurotransmitters in the central nervous system of mammals and plays a very important role in the functioning of the central nervous, hormonal and cardiovascular systems. Many diseases are related to the change of catecholamine concentration, therefore it is necessary to develop quantitative methods for the determination of catecholamine concentration in order to study its physiological effect and to have the possibility to diagnose some diseases in the clinical medicine field. In recent years, many methods have been reported for the determination of EN. However, most of these methods are complicated because they need derivatization or combination with various detection methods. Also, some of them have low sensitivity, and low selectivity.
Ascorbic acid (AA), is a water soluble vitamin which widely present in both animal and plant kingdoms. It is a vital component in the human diet and in multivitamin preparations. It helps to maintain the elasticity of the skin, aids with the absorption of iron and improves resistance to infection. Also, AA is a powerful antioxidant and an effective reducing agent in food products, preventing change in color, odour and taste, as well as extending the storage time of the products.
However, it is almost impossible to detect simultaneously these components using direct spectrophotometric method by oxidation with heteropoly compounds (HPC), because these compounds reduce HPC under the same conditions, which leads to the acquisition of total optical density of the solution.
There was carried out the study of oxidation rate of AA and EN by 16-molybdo-2-tungstodiphosphate at different acidity. We also checked the influence of Cu(II)-ions on the rate of the above-mention reaction.
|
Fig. 1 Time dependence of optical density of HPC solutions with AA and EN under different conditions.СHPC = 9,76·10-5 М, СAA = СEN = 2,44·10-5 М, СCu = 9,76·10-4 М. l = 2 сm, л = 790 (рН 3,9) nm, л = 820 (рН 2,8) nm. |
According to the Fig., it is possible draw conclusion that with the increase in acidity the reducing rate of HPC decreases and vice versa. The addition of the solution of Cu(II) facilitates the increase in oxidation rate. At the pH 2,80 and along with the addition of Cu(II)-ions in reaction mixture the conditions for determining the components by H-Point method are theoretically observed.
The according data prove the possibility of development of the spectrophotometric method of simultaneous determination of AA and EN using the kinetic H-Point method. This method allows to estimate AA and EN quantitively in real samples without using complicated and rather expensive methods, such as liquid chromatography.