Diuzheva A. I., Zhuk L. P., Posudiievska O. R.
Oles Honchar Dnipropetrovsk National University
DETERMINATION OF TRIADIMEFON IN NATURAL WATERS
The main problem of nowadays is water pollution by pesticides. At present time the determination of organic substances, which belong to different classes of pesticides, is carried out by gas chromatography methods in natural objects. As an alternative, we propose a spectrophotometric method for determination of triadimefon in the water.
For the development of methodology for determination of triadimefon in natural water we studied its reaction with azodye magneson XS (MG) in the presence of cationic flocculant Puro Flock 920 (PF) and the ions of Cu (II).

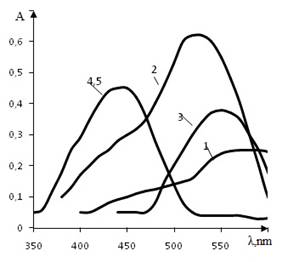
Fig. 1
Adsorption spectrum of MG (1), МG-PF (2), МG-Сu(II) (3), МG-PF-TD (4), МГ-PF-TD-Cu(II) (5), рН 4; СМG=2,56·10-5 mol/L; СPF=1,6·10-6 mol/L; CCu,mol/L: 3 – 4·10-8, 5 – 4·10-4; СTD, mol/L: 4 – 8·10-6, 5- 8·10-7;Specord М-40, l=1,0 cm
In the range of pH 3–5, the ion associate of PF with H2R2- form of azodye is created, with the maximum of the absorption band at 520 nm. With increasing of pH hypochromic shift is growing to 100 nm. Values of pH ½ = 6,18 and 7.52 show the influence of PF on the tautomeric equilibrium of the dye (pK = 8,62). At pH 4 in the presence of TD the spectrum of the dye does not change, however in the presence of Cu (II) the optical density decreases. In the solution of MG-PF-TD the absorption band shifts hypochromically to 450 nm (Δλ=100 nm) (fig. curve 4), CTD = 8·10-6 mol/L, ε=5,2·104). The introduction of Cu (II) ions to the solutions allows to increase the sensibility of the reaction (СTD=8·10-7 mol/L, ε =5·105) (fig. curve 5).
Visually the color changes from red to orange. This effect is achieved by immobilization of interacting particles due to Cu (II) on the polymer chain.
Sensitive reaction of triadimefon with MG and PF at pH 4 can be recommended for identification of high concentrations of triadimefon (10-6 – 10-7 mol/L), in the presence of Cu (II) ions in low concentrations (10-7 – 10-8 mol/L) in natural waters.
The presence of fulvic and humic acids, as well as ions of Ca (II), Mg (II), Fe (III), Ni (II), Co (II) etc., does not influence on the determination of triadimefon in natural waters.